Reaction-diffusion textures come from a set of coupled partial differential equations that result in appealingly cellular, organic solutions.
Turk[Turk1991] quotes these as Turing's original [Turing1952], discrete 1D reaction-diffusion equations, which relate the concentrations of two chemical species ![]() and
and ![]() , discretized into cells
, discretized into cells ![]() and
and ![]() .
.
Here ![]() is the reaction rate parameter,
is the reaction rate parameter, ![]() and
and ![]() are diffusion rate parameters, and
are diffusion rate parameters, and ![]() is a decay parameter for
is a decay parameter for ![]() .
We can interpret
.
We can interpret ![]() as a chemical, generated in barren
regions, that is consumed to produce chemical
as a chemical, generated in barren
regions, that is consumed to produce chemical ![]() .
. ![]() naturally decays, with an even higher decay rate where the concentration of
naturally decays, with an even higher decay rate where the concentration of ![]() is large. Both species diffuse, but
is large. Both species diffuse, but ![]() normally diffuses faster than
normally diffuses faster than ![]() .
.
Turk recommends ![]() of approximately 12, but picks a random slightly different value for each cell. We note nicely random results can be obtained even with fixed parameters by randomly varying the initial conditions--we found favorable results using initial values for
of approximately 12, but picks a random slightly different value for each cell. We note nicely random results can be obtained even with fixed parameters by randomly varying the initial conditions--we found favorable results using initial values for ![]() and
and ![]() randomly distributed on the interval
randomly distributed on the interval ![]() . Turk recommends a diffusion rate of
. Turk recommends a diffusion rate of ![]() (the reaction-free stability limit) and
(the reaction-free stability limit) and ![]() . We find these parameters work well.
. We find these parameters work well.
We note that the equation for ![]() is unstable if
is unstable if ![]() is negative. Since negative results can occur because of the fixed decay factor,
is negative. Since negative results can occur because of the fixed decay factor, ![]() must be clamped to zero or the solution grows without bound.
must be clamped to zero or the solution grows without bound.
We can easily derive the multidimensional and continuous--in both space and time--version of Turing's reaction/diffusion equation. Note that we have generalized the fixed ![]() growth constant 16 to
growth constant 16 to ![]() .
.
Here ![]() is the reaction rate parameter,
is the reaction rate parameter, ![]() and
and ![]() are diffusion rate parameters,
are diffusion rate parameters, ![]() is a growth parameter and
is a growth parameter and ![]() is a decay parameter. We always use the values
is a decay parameter. We always use the values ![]() ,
, ![]() , and
, and
![]() , since these values result in spatial features that are a few units across.
, since these values result in spatial features that are a few units across.
In practice, we must (re)discretize this continuous set of equations in both space and time in order to solve this system. Using the first-order Euler method, this recovers Turing's original discrete equations--that is, these continuous equations can be seen as a way to derive consistent parameters for the discrete equations. For a timestep of ![]() ,
,
![]() . For a uniform mesh size
. For a uniform mesh size ![]() , in 2D
, in 2D
![]() and similarly
and similarly
![]() . We of course always choose
. We of course always choose ![]() as large as possible--the theoretical diffusion stability criterion requires
as large as possible--the theoretical diffusion stability criterion requires ![]() ; and the nonlinear reaction rate also has some limit, which appears to be near
; and the nonlinear reaction rate also has some limit, which appears to be near ![]() .
.
The advantage of using a continuous formulation is that we can take advantage of the powerful tools available for quickly solving partial differential equations, such as the multigrid method. For small grid spacings and large domains, multigrid can dramatically accellerate convergence, as illustrated in Figure 1. We find the coarse-to-fine direction of multigrid to be sufficient--no reverse restriction is required.
| ||||
|
Figure 1. Illustrating the dramatic speed advantages of
multigrid on a 1024x1024 domain with a grid spacing of |
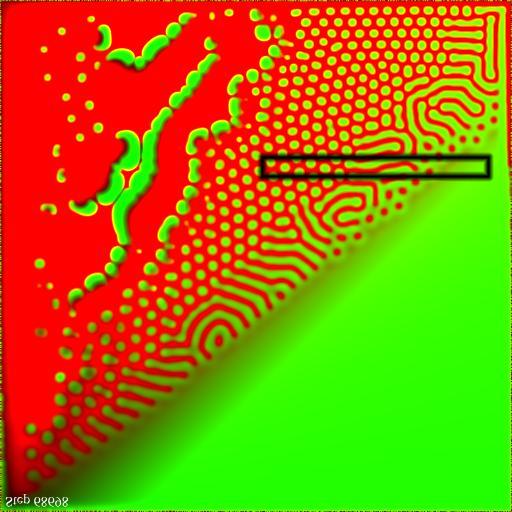
The character of the solutions to this equation depends strongly on the ratio ![]() , as illustrated in the parameter map below. Interesting, spatially varying results only appear for
, as illustrated in the parameter map below. Interesting, spatially varying results only appear for ![]() near one. Larger values over-active, which results in
near one. Larger values over-active, which results in ![]() dominating; while smaller values fail to achieve a self-sustaining quantity of
dominating; while smaller values fail to achieve a self-sustaining quantity of ![]() , which results in unstable pulsating patterns. In the remainder, we fix
, which results in unstable pulsating patterns. In the remainder, we fix ![]() at 20 and vary the growth rate
at 20 and vary the growth rate ![]() between 17.5 and 27.5, as shown in the dark box.
between 17.5 and 27.5, as shown in the dark box.
 |
|
Figure 2.Parameter map for growth and decay parameters. The growth rate |
| ||
| Figure 3.Varying growth parameter according to a photograph for a bizarre effect. |
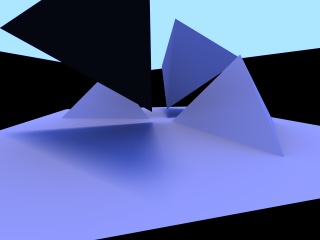 Globally illuminated scene without texture. |
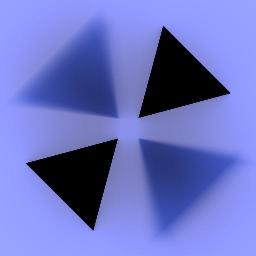 Illumination on ground plane. |
 Growth rate derived from illumination. |
 Grown texture. |
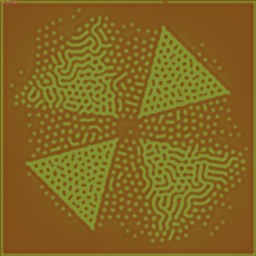 Grown texture, recolored. |
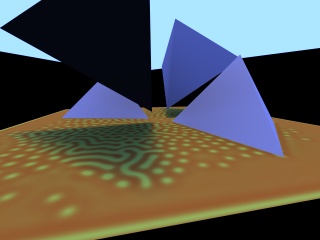 (900K MPEG Movie, 320x240) Scene rendered using grown texture. |